SAMPLES CAN BE DIFFICULT, BUT MEASUREMENT CAN BE EASY.
The pH value is a crucial parameter for many applications.
It is a fundamental parameter in the food industry, for optimizing nickel or copper plating, for ensuring optimal conditions for healthy plant growth and many more.
The reason is that the pH value affects many biological and chemical processes like proliferation/survival of microorganisms, the activity of enzymes, chemical reactions and equilibria, solubility of salts, etc.
Most often (and also most accurately) the pH value is measured by a pH meter with a glass electrode. Receiving right and stable values can however be difficult, especially in some samples (like food, soil, creams, paint, samples with high pH, etc.).
It is important to understand what pH means, how a pH electrode works and also how measuring can be easier by using the right electrode.
Hanna Instruments is a leading pH electrode manufacturer and offers a great variety of electrodes designed for specific applications.
Petrochemical industry
1. What is pH?
The pH value is a measurement of how many active hydrogen ions are present in a sample. In practice, concertation is used more often instead of activity.
In simpler words, pH is a measurement of how acidic or how alkaline an aqueous solution is.
The term ‘’pH’’ stands for “pondus hydrogenii” or “the potential of hydrogen” and it was first introduced by the Danish biochemist, Soren Peter Lauritz Sorensen, in 1909.
During his research, he often needed to measure how acidic is the sample and found it more convenient to write the concentration of hydrogen ions in the decadic logarithmic scale (for example 0.0000001 mol/L H+ becomes pH 7). pH has been then defined as [1]:


a = activity
c = concentration
H+ = hydrogen ion
The pH value is thus measured on a logarithmic scale from 0 to 14, with 7 being neutral (Picture 1).
When a substance called acid is added to water, it increases the concentration of hydrogen ions and the pH values falls below 7. Conversely, a substance called base decreases the concentration of hydrogen ions and the pH value rises above 7. This means, that every solution below pH 7 is acidic, and every solution above 7 is alkaline. Since we use the decadic logarithm, 1 pH less means a 10 times increase of the activity of H+. [2]


2. How does a pH electrode work?
A glass electrode or a pH electrode is a type of an ion-selective electrode – it is selective for measuring hydrogen ions. The following parts are needed for potentiometric pH measurement:
- reference electrode, most common is the silver chloride half-cell (silver wire coated with AgCl in contact with saturated KCl solution) – has constant potential
- indicator electrode with the pH sensitive glass – changes potential in response to the concentration of the analyte,
- mV meter – reads the difference in potential between the reference and indicator electrode.
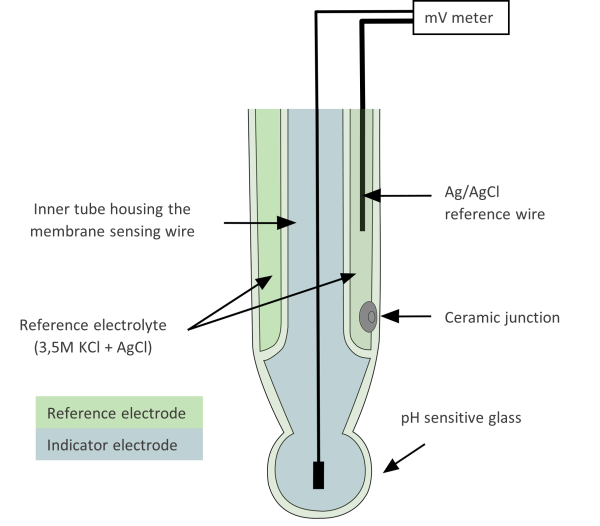

Usually, a combination pH electrode is used (Picture 2), that incorporates both the reference and the indicator electrode.
The part of the electrode that is sensitive to pH is the thin glass bulb or cone at the bottom of the indicator electrode. On the surface of this sensitive glass are negatively charged oxygen atoms (from SiO4) that can bind positively charged ions of a suitable size – hydrogen ions (and also alkali ions but with less strength).
How many hydrogen ions are bonded to the surface of the sensitive glass depends on the activity (concentration) of the hydrogen ions in the sample. When hydrogen ions bond to the pH sensitive glass, an electric potential is generated. Therefore, by reading this potential with a mV meter, the pH value can be calculated using the Nernst equation [2]:
E = potential in mV
E0 = electric potential produced by the reference electrode
β = the effect of dehydration, contamination, scratches etc. on the glass membrane
Another crucial component of an electrode is the junction, which allows contact between the solution in the reference electrode (the electrolyte, saturated KCl) and the sample. The electrolyte leaks out of the reference electrode into the sample, closing the electrical circuit and making measurements possible. The greater the flow of the electrolyte, the better the response of the electrode. [2]
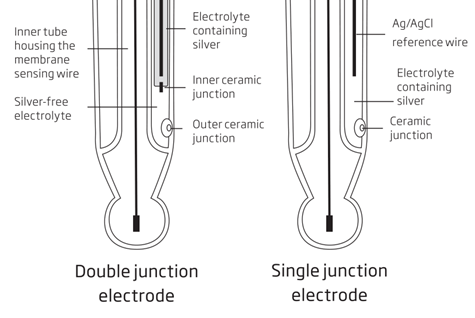
Standard single junction electrodes have the reference electrode in direct contact with the sample (Picture 3). Silver chloride present in the reference electrode may have low solubility in the sample, thus when making contact with the sample, precipitate forms on the external face of the junction resulting in drifty readings. Under adverse conditions (high pressure, high temperature, high conductivity) the flow of the electrolyte can also be reversed, resulting the contamination of the refence half-cell and complete electrode failure.
Double junction electrodes have, as the name implies, two junctions (Picture 3). These electrodes have an extra compartment for the reference electrode with the inner junction. The intermediate area is silver-free and not sensitive to contamination, increasing the longevity of the electrode.

Why pH electrodes need to be calibrated?
The E0 and β values in the Nernst equation depend on the state of the glass membrane (level of hydration, deposits, scratches, etc.) and the junction (level of clogging).
Calculating these values would be impossible in practice, and changes are too significant for the results. After calibrating the pH electrode with solutions of a known value (preferably with at least two), measurements are adjusted accordingly.
Why pH electrodes need to be maintained?


Hydrogen ions also cannot bind to the surface, if there are (organic or inorganic) deposits. The amount of surface that is available for boding affects the quality of the measurements.
Cleaning with the HI7061 general purpose or an application specific cleaning solution will remove the deposits and restore the electrode (Picture 5).


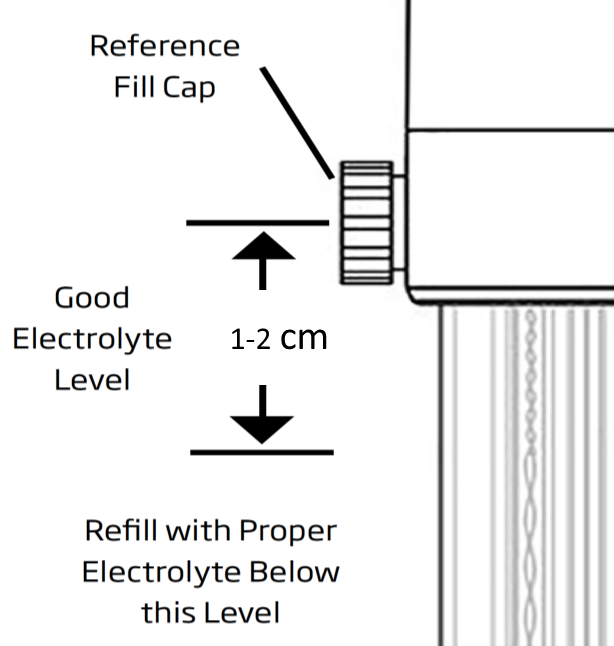

The level of the electrolyte in the reference electrode should be kept at 1-2 cm below the fill hole for maintaining appropriate pressure for electrolyte outflow (Picture 6).
Before measurements, the electrolyte levels should be checked and refilled with HI7082 electrolyte for double junction electrodes or HI7072 electrolyte for single junction electrodes if needed. And during measurements, it is important that the cap is loose or open.
How to check the state of the pH electrode?
To check the electrode, if it is in a good condition, the offset and the slope should be determined.
The offset is the mV value measured in a solution with pH 7.0. Theoretically, this value should be 0 mV, but in practice ± 30 mV offset is acceptable.
The slope can be determined by measuring the mV value in solutions with pH 7.0 and pH 4.0 (or pH 10.0) and calculating the absolute mV difference.
From the Nernst equation we can see that this difference should be 59,16 mV * 3 (the difference in pH) = 177,48 mV, but from 150 mV to 186 mV is an acceptable slope.
3. Measuring pH of difficult samples
When measuring pH with a glass electrode, we may face some difficulties. First, we have to make sure that the electrode has been properly maintained (cleaned, hydrated, refilled) and calibrated with fresh buffers. If problems persist, then the design of the electrode is not suitable for measuring pH in our sample. Some of the most common problems are:
- when there is not enough electrolyte flow due to chemical/physical properties of the sample or junction clogging,
- when the sample is semi-solid or solid,
- when there is a significant concentration of sodium or other alkali ions,
- if we are dealing with a sample with HF,
- if we are measuring samplers with high/low temperatures or
- when there is noise from the environment.
Hanna Instruments offers solutions to all these problems with special junctions, tips, glass type and body designs.
3.1. Low electrolyte flow
Examples:
- low conductivity samples (e.g., drinking water, samples with less than 100 μS/cm)
- high conductivity samples (e.g., brines, seawater, strong acids, strong bases)
- viscous samples (e.g., paint, cosmetics, pastes, emulsions)
Having a sufficient electrolyte flow from the reference electrode into the sample through the junction is crucial for receiving stable readings.
In cases when the reference electrolyte and the sample differ greatly in composition, this flow is limited and a phenomenon called junction potential has a significant effect on measurements.
Standard electrodes have one ceramic junction that does not provide sufficient electrolyte flow when pH of low conductivity samples, high conductivity samples or viscous samples is measured. By using electrodes with multiple ceramic junctions, the flow rate is increased and readings stabilized.
Hanna Instruments HI10530 double junction pH electrode with a triple ceramic junction on the outer junction (Picture 7) is designed for reliable pH measurements in low conductivity and viscous samples, featuring an electrolyte flow rate of 40 to 50 µL/hour (compared to 15 to 20 µL/hour for a standard electrode).
Furthermore, the low temperature glass type with lower impedance improves the quality of pH measurements in such samples.
Hanna Instruments HI10430 double junction pH electrode with double ceramic junction (Picture 8) is ideal for applications involving high conductivity samples or concentrated samples, having the electrolyte flow rate of 30 to 40 µL/hour.
The double junction system also shields the reference electrode from contamination.
3.2. Clogged junction
Examples:
- “dirty” samples high in solids (e.g., wine, juice, mash, wort)
- fat
- protein content (e.g., milk, meat, cheese and other food samples)
Like previously discussed electrolyte leaking from the reference electrode into the samples closes the electrical circuit and makes measurements possible. Any clogging of the junction will result in erratic and unstable readings.
“Dirty” samples that have a high content of solids, fats, proteins etc. can quickly clog the conventional ceramic junctions and make the electrode unusable.
Choosing an electrode with a special junction design that is resistant to clogging (e.g., Clogging Prevention System, cloth junction or open junction) prolongs the longevity of the electrode.
Hanna Instruments HI1048 pH electrode features the Clogging Prevention System (CPS) technology (Picture 9) is designed for pH analysis of wine, must and juice.
The CPS technology utilizes the porousness of ground glass coupled with a PTFE sleeve to prevent clogging of the junction.
The ground glass allows the proper flow of the liquid, while the PTFE sleeve repels solids.
Another junction design fit for measuring the pH of samples with high content of soils is the retractable cloth junction.
In this case the junction can be cleared by simply extracting approx. 3 mm of the junction.
Hanna Instruments FC214 pH electrode (Picture 10) features the cloth junction and is ideal for analysis of mash and wort in the beer making process.
Hanna Instruments Foodcare series pH electrodes feature the open junction design.
In this type of junction, where a solid gel interface (viscolene) is between the sample and internal Ag/AgCl reference.
This interface makes it impermeable to clogging, resulting in a fast response and stable readings.
Additionally, this kind of junction prevents sample contaminations that is crucial when testing food.
3.3. Solid and semi-solid samples
Examples:
- semi solid samples (e.g., yogurt, cheese, meat, creams, soil samples)
- solid samples (e.g., skin, leather, agar plates, paper)
The conventional spherical shaped pH electrodes were designed for watery samples since it provides a large surface for sample interaction.
With this type of tip shape, it is however difficult to penetrate into semi-solid samples or make contact with solid samples. Electrodes with conical or flat shaped tips make direct measurement possible.
Testing of semi-solid samples has been made easy with conical shaped tip electrodes. This type of tip design allows for penetration and direct measurement of pH in samples like yogurt, cheese, meat, creams, soil etc.
Hanna Instruments offers a great variety of conical shaped electrodes designed for specific samples (Picture 11).










Picture 11: Hanna Instruments electrodes
In case of solid samples, when penetration into the sample is not possible, surface contact should be optimized using a flat shaped electrode.
Hanna Instruments HI1414 electrode with a flat shaped tip (Picture 12) is designed for surface measurements of pH on skin, leather, agar plates, paper, etc.
3.4. High pH samples
Examples:
- samples with pH higher than 12 (e.g., detergents, soapy water, household cleaners, strong bases)
The pH sensitive glass is not strictly selective for hydrogen ions.
Alkali ions, especially sodium ions, can also bind to the glass surface, but with less strength and in most cases do not have a significant effect on pH measurements.
In samples with high pH, the concentration of hydrogen ions is very small compared to the concentration of alkali ions and the amount of alkali ions boded to the glass surface makes significant changes in the read potential.
The displayed pH value is therefore lower than it actually is. The difference between the theoretical and measured values is called alkaline error.
Hanna Instruments high temperature glass minimizes alkali error in highly alkaline solutions.
At pH 13 in a solution with 1,0M sodium concentration the alkaline error for a high temperature glass is 0,15 pH, in comparison to 0,43 pH for general purpose glass and over 0,79 pH for low temperature glass.
3.5. Samples with hydrofluoric acid
Examples:
- laboratory reagents and analytical standards containing fluoride
Hydrofluoric acid can dissolve glass rapidly. Hanna Instruments HI1143 electrode uses HF resistant glass for aggressive applications that include fluoride ions (up to 2 g/L).
Electrodes manufactured with this glass last ten times longer than electrodes made with standard pH glass formulations (from 10 to 100 days). Additionally, the double junction design protects the reference electrode contamination.
3.6. High/low temperature samples
Examples:
- cooled food
- pasteurization
- wort boil
At low temperatures electrodes with a high impedance glass will give at low temperature very noisy and erratic signals. Hanna Instruments electrodes with LOW temperature glass have low impedance and enable stable measurements at lower temperatures.
At elevated temperatures, however, the glass can dissolve readily, shortening the life and performance of the electrode. Hanna Instruments electrodes with HIGH temperature glass are designed for extended use at elevated temperatures. With higher resistance glass measurements at elevated temperatures are accurate with excellent response times.
3.7. Samples with electrical currents
Examples:
- galvanic baths
- cooling towers
- boilers
- swimming pools
Electrical currents in the sample can affect the reference half-cell voltage that is connected via the liquid junction with the sample. In this case, the reference electrode picks up the electromagnetic fields and the measurement of the pH is altered.
A matching pin is a differential measurement technique used to eliminate ground loops and common mode perturbations for a measurement system, thus isolating current/magnetic fields from the reference electrode. Hanna manufactures a number of models with the matching pin design for safe precise pH measurements.
This problem can also be solved by using titanium bodied electrodes (Picture 13). The titanium cage acts like a Faraday cage and shields the inner part of the electrode from the outside noise.
Not sure which electrode would be the best for your sample?
Contact a Hanna Technical Specialist at info@hannaservice.eu or using our contact form.
REFERENCES:
- Jensen, William B. (2004). “The Symbol for pH” (PDF). Journal of Chemical Education.
- Harris C. Daniel (2010). Quantitative Chemical Analysis, 8th Edition. H. Freeman and Company, New York


With Great Product
Come Great Results









