Wine production involves complex chemical reactions and one crucial aspect is the measurement of reducing sugars in the wine.
Reducing sugars play a significant role in the fermentation process, affecting the taste, aroma and overall quality of the final product.
Accurate and reliable measurements of reducing sugars are essential for winemakers to monitor fermentation progress, determine the appropriate time for harvesting grapes and ensure consistent product quality.
This article aims to compare the photometer and titration techniques, evaluating their accuracy, precision, advantages and limitations to assist winemakers in selecting the most suitable method for measuring reducing sugars in wine.
1. Introduction to Measuring Reducing Sugars in Wine
Importance of Measuring Reducing Sugars
When it comes to wine production, measuring reducing sugars is a crucial step in determining the sweetness of the final product. The level of reducing sugars directly affects the taste and overall quality of the wine. Therefore, accurately measuring these sugars is essential for winemakers to ensure consistency and meet consumer expectations.
Role of Reducing Sugars in Wine Production
Reducing sugars play a significant role in wine production as they act as a food source for yeast during fermentation. As yeast consumes these sugars, it produces alcohol and carbon dioxide, resulting in the conversion of grape juice into wine. The concentration of reducing sugars in the initial grape juice determines the potential alcohol content of the finished wine.
Previous Methods for Measuring Reducing Sugars
In the past, winemakers would rely on labor-intensive and time-consuming methods, such as the Lane-Eynon method, to measure reducing sugars in wine. This method involved multiple steps and required the use of chemical reagents, making it less convenient and prone to human error. However, advancements in technology have introduced more efficient and accurate methods, such as photometry and titration.
2. Photometer as a Method for Measuring Reducing Sugars
Principle and Mechanism of Photometer
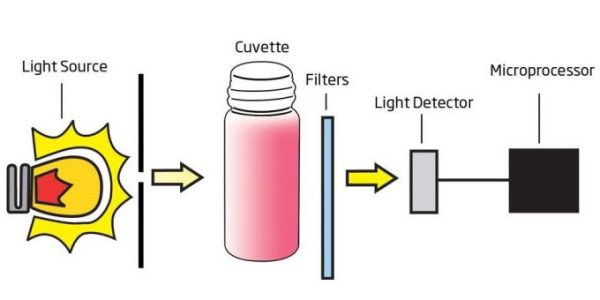
A photometer is a device that measures the absorption of light by a sample, allowing for the quantification of reducing sugars in wine. It works on the principle that reducing sugars react with specific reagents to produce a color change. The photometer then measures the intensity of light absorbed by the sample, which correlates with the concentration of reducing sugars.
Benefits of Using Photometer for Measuring Reducing Sugars
Using a photometer offers several advantages for measuring reducing sugars in wine. Firstly, it provides a quick and easy method that eliminates the need for complex chemical reactions. Additionally, a photometer offers high precision and accuracy, reducing the potential for human error and ensuring reliable results. It also allows for a wide range of wine samples to be analyzed with minimal sample preparation.
Steps Involved in Using a Photometer
To measure reducing sugars using a photometer, a winemaker simply needs to mix a wine sample with a reagent that reacts with the sugars. After a specific incubation period, the sample is inserted into the photometer, which measures the absorbance of light. The photometer then calculates the reducing sugar concentration based on the absorbance values, providing a quick and reliable measurement.
HI83746-02
Photometer for Reducing Sugars in Wine
The HI83746 Photometer for Reducing Sugars in Wine combines accuracy and ease of use in an ergonomic, portable design.
A user can accurately determine the concentration of reducing sugars in wine within a 0.00 to 50.0 g/L (ppt) range using the HI83746-20 ready-made reagents.
- Built-in Timer
- Automatic Shut Off
- Ready-made Reagents
The HI83746 uses the Fehling method to determine the concentration of reducing sugars less than 50.00 g/L (ppt).
When Fehling’s A and Fehling’s B Solutions react with a sample containing reducing sugars, the sample will undergo a color change; the greater the concentration, the deeper the color. The associated color change is then colorimetrically analyzed according to the Beer-Lambert Law. This principle states that light is absorbed by a complementary color and the emitted radiation is dependent upon concentration. For determination of reducing sugars, a narrow band interference filter at 610 nm (orange) allows only orange light to be detected by the silicon photodetector and omits all other visible light emitted from the tungsten lamp. As the change in color of the reacted sample increases, absorbance of the specific wavelength of light also increases, while transmittance decreases.

3. Titration as a Method for Measuring Reducing Sugars
Principle and Mechanism of Titration
Titration is another method commonly used to measure reducing sugars in wine. It involves gradually adding a reagent, known as a titrant, to a wine sample until a reaction endpoint is reached. In the case of reducing sugars an excess of copper II is added to a wine sample, the sample is then heated to causing the residual sugars to reduce the copper II to copper I. The excess copper II is treated with potassium iodide to form iodine. The iodine is then titrated with sodium thiosulfate.
Benefits of Using Titration for Measuring Reducing Sugars
Titration has been a traditional method for measuring reducing sugars and offers its own set of advantages. It is a widely accepted technique with established protocols, making it familiar to many winemakers. Titration also allows for the simultaneous measurement of other wine parameters, such as acidity, providing a comprehensive analysis.
Steps Involved in Titration Analysis
To perform titration for reducing sugars, a winemaker needs to prepare a wine sample and add a titrant, such as Fehling’s solution, drop by drop until reaching endpoint. The number of drops required to reach the endpoint is then used to calculate the concentration of reducing sugars in the wine sample.
HI931-02
Automatic Potentiometric Titrator
The HI931 Automatic Titrator is the answer to your dedicated titration needs. Fully customizable, the HI931 delivers accurate results and intuitive user experience, all in a compact package. Titrate for a variety of measurements at the push of a button including acids, bases, redox, and selective ions. No additional programming upgrades to purchase. The only things you need to start using the HI931 are a sensor and titrant.
- Small footprint so you can fully optimize your benchtop
- Unmatched 40,000-step dosing pump for small volumes of titrant to help you achieve a very precise endpoint for greater consistency
- Flexibility to store up to 100 methods
4. Comparison of Photometer and Titration Techniques
Precision and Accuracy of Photometer
When comparing the two methods, the titrator generally offers higher precision and accuracy due to its automated measurement process. The digital readings provided by the photometer minimize human error and provide more consistent results, ensuring reproducible measurements.
Precision and Accuracy of Titration
Titration also provides results with high accuracy, the nature of the technique introduces a higher potential for human error. An important consideration when deciding on an automatic titrator is how many titrations we need to do in a given time and how much time we want to save for other work in the laboratory.
Automatic titrations can be done in under three minutes. By simply pressing the ‘’START’’ button, the automatic titrator will begin the titration. In the meantime, we can do other work in the laboratory (like preparing the next sample), and when we return, the results will be waiting for us on the display.
5. Accuracy and Precision of Photometer and Titration Methods
Factors Affecting the Accuracy of Photometer Measurements
When it comes to measuring reducing sugars in wine, accuracy is key. Factors that can affect the accuracy of photometer measurements include the quality of the photometer itself, the calibration of the instrument and the handling of the samples. It’s important to ensure that the photometer is properly calibrated and that the samples are prepared and measured according to the manufacturer’s instructions.
Factors Affecting the Precision of Photometer Measurements
Precision refers to the consistency and reproducibility of measurements. In the case of photometer measurements, factors such as sample preparation, operator technique and any fluctuations in the light source can impact precision. It’s essential to follow the recommended procedures carefully and minimize any sources of variability to achieve reliable and precise results.
Factors Affecting the Accuracy of Titration Measurements
Titration is a classic method for measuring reducing sugars in wine, but it also has its own set of factors that can affect accuracy. The accuracy of titration measurements depends on the quality and concentration of the reagents used, as well as the proper handling and execution of the titration procedure. It’s crucial to have fresh and correctly standardized reagents to ensure accurate results.
Factors Affecting the Precision of Titration Measurements
Precision in titration measurements can be influenced by factors such as the skill and technique of the operator, the use of appropriate measuring equipment, and the consistency in performing the titration procedure.
6. Advantages and Limitations of Photometer and Titration Methods
Advantages of Photometer Method
The photometer method offers several advantages over titration. The use of a photometer eliminates the need for potentially hazardous reagents and complicated titration setups. It also allows for easy data recording and analysis, making it a convenient choice for routine analysis.
Limitations of Photometer Method
While the photometer method is fast and easy to use, it does have some limitations. The accuracy of measurements can be dependent on the specific instrument used and its calibration. Additionally, the photometer method may not be suitable for samples with complex matrices or high levels of interfering substances. In such cases, alternative methods like titration might be more appropriate.
Advantages of Titration Method
Titration has stood the test of time as a reliable method for measuring reducing sugars in wine. It offers excellent accuracy when performed correctly and can handle samples with complex matrices. The titration method also allows for adjustments during the process, ensuring precise and targeted results. It remains a preferred choice for many researchers and professionals in the field.
Limitations of Titration Method
7. Practical Considerations for Choosing the Appropriate Method
When it comes to selecting the appropriate method for measuring reducing sugars in wine, several practical considerations come into play. Firstly, consider the specific requirements of your analysis, such as the desired accuracy, precision and sample throughput. If accuracy and flexibility are of utmost importance, titration could be the preferred option.
Consider the complexity of your sample matrix and the potential for interfering substances. If your wine samples are known to have high levels of interferences, titration might be more suitable due to its ability to handle such matrices. However, if you are working with relatively simple sample matrices, the photometer method could be a viable choice.
Lastly, evaluate the available resources, including the equipment, reagents and personnel expertise.
Ultimately, the choice between the photometer and titration methods depends on a variety of factors specific to your needs. Consider the advantages and limitations of each method, as well as the practical considerations, to make an informed decision that best suits your requirements.
8. Conclusion and Recommendations
After a comprehensive comparison between the photometer and titration techniques for measuring reducing sugars in wine, it is evident that both methods have their advantages and limitations.
Photometry offers greater precision and ease of use, while titration provides a more traditional approach with wider applicability.
Winemakers should consider their specific needs, resources, and desired level of accuracy when choosing the appropriate method.
Ultimately, by accurately measuring reducing sugars, winemakers can enhance their control over fermentation processes, improve wine quality, and ultimately delight consumers with exceptional wines.
Have questions?
Contact a Hanna Technical Specialist at info@hannaservice.eu or using our contact form.
AUTHOR:
Tajana Mokrović, mag.nutr.