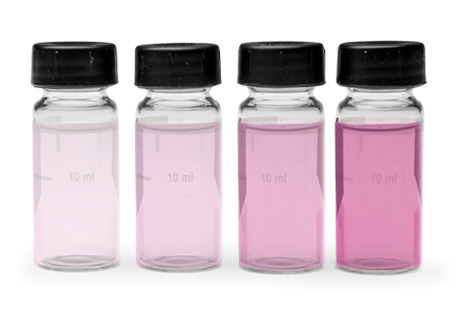
FIFTY
SHADES
OF
PINK
SHADES
OF
PINK
In most cases free chlorine residual is determined through DPD “magenta colored” test results. The method is standardly reliable and values easily projected.
The US Environmental Protection Agency-accepted colorimetric N,N-diethyl-pphenylenediamine (DPD) method is the most commonly used procedure to determine free chlorine residuals in water.
The DPD indicator immediately reacts with free available chlorine—hypochlorous acid or hypochlorite ion—to form a pink color, which is proportional to the chlorine concentration. However, some DPD test results may be misleading because monochloramine residual interferes with DPD free analysis, creating a false-positive reading.

The principal reactions for
chloramine formation are
presented below:
chloramine formation are
presented below:
HOCl + NH3 → NH2Cl + H2O
HOCl + NH2Cl → NHCl2 + H2O
HOCl + NHCl2 → NCl3 + H2O
HOCl + NH2Cl → NHCl2 + H2O
HOCl + NHCl2 → NCl3 + H2O
The DPD free chlorine reagents may develop a false reading that ranges from faint pink (0.1–0.3 mg/L) immediately to dark magenta (1.0+ mg/L) over time, depending on how much monochloramine is present in the water sample.
HI97711
Free and Total Chlorine Portable Photometer
The HI97711 Free and Total Chlorine Photometer combines accuracy and ease of use in a simple, portable design. The advanced optical system provides lab-quality accuracy while its user-friendly design is easy for any user making it the perfect photometer for your water quality testing needs. The HI97711 meter measures free and total chlorine in water samples from 0.00 to 5.00 mg/L (ppm).
- No warm up time needed before taking a measurement.
- Tutorial mode for easy step-by-step instructions.
- Uses either powder or cost saving liquid reagents.
AVOIDING THE ERROR
IN YOUR READING

To determine chloramine or combined chlorine concentration, the total chlorine DPD method contains a special ingredient potassium iodide in the reagent packet with the DPD indicator.
Chloramine converts the iodide reagent to iodine, which reacts with DPD to form a pink magenta color.
After analysis, subtracting the free chlorine test results from the total chlorine results yields the combined chlorine concentration.
However, if the free chlorine results are false positive, the calculation is flawed.
Checklist for free chlorine residual determination:
- When you add your DPD free chlorine reagents into the water sample, do the reagents turn a faint pink color, and gradually color develops more intensively?
- Do you wait 1 min or longer for the pink color to develop further, ensuring that you register more free chlorine residual?
- Do you get different results in subsequent measurements?
- Do you have a record of ammonia presence in your water supply and distribution system?
- Do you have trouble maintaining stable chlorine residuals across your distribution system?
- Do you detect a chlorine odor in the treated water?
- Is the chlorine mg/L dosage rate much higher than the free residual results obtained?
If you encountered any of the situations, you may have insufficient amount of free chlorine residual :
Combined monochloramine interferes with the free chlorine DPD analysis and creates a false positive reading that’s artificially higher than reality. Compare the total chlorine DPD method results with your free chlorine residual and then determine the combined chlorine residual.
Total chlorine residual should consist of > 85 percent free chlorine.
Free chlorine DPD test reagents react immediately and shouldn’t take time to develop color.
Color drifting to darker pink is the first warning sign of chloramine interference!
Color drifting to darker pink is the first warning sign of chloramine interference!
To avoid interference from combined chloramine residual, free chlorine residual should be measured within 1 min.
Ammonia Contamination
Ammonia (NH3-N) can occur naturally as contaminant often found in well sourced groundwater supplies and seasonally found in surface water supplies.
Typical sources of ammonia contamination in water:
- fertilizer runoff
- municipal effluent discharges
(sewage, septic tanks) - decay of natural deposits
- xcretion of nitrogenous wastes
from animals
Ammonia (NH3-N) can occur naturally as contaminant often found in well sourced groundwater supplies and seasonally found in surface water supplies.
Typical sources of ammonia contamination in water:
- fertilizer runoff
- municipal effluent discharges
(sewage, septic tanks) - decay of natural deposits
- xcretion of nitrogenous wastes
from animals

Although naturally occurring ammonia doesn’t create a direct cosmetic or aesthetic problem for consumers, it works as interference in Free Chlorine readings.
Ammonia (NH3 -N) presence also creates a greater demand on chlorine chemicals and thus enables bigger chemical waste and economic loss.
About 10 mg/L of chlorine is required to consume and destroy 1 mg/L of ammonia-N before true free chlorine residual is formed.
To determine the cause of the problem, analyze raw and treated water for ammonia:
HI97715
Ammonia Portable Photometer (MR)
The HI97715 Ammonia Photometer combines accuracy and ease of use in a simple, portable design. The advanced optical system provides lab-quality accuracy while its user-friendly design is easy for any user making it the perfect photometer for your water quality testing needs.
- The HI97715 measures ammonia in water samples from 0.00 to 10.00 mg/L (ppm).
Without ammonia contamination in their water supply, operators can maintain optimal free chlorine residuals (85 percent of total residual) in their water system, resulting in less chlorinous odor.
*Besides monochloramine, pressence of other oxidative substances can also interfere with measurements giving a false positive reading: Bromine (Br2), Chloride dioxide (ClO2), Iodine (I2), Oxidized Manganese and Chromium, Ozone (O3).
Author:
Nives Vinceković Budor
mag.ing.chem.ing.
Nives Vinceković Budor
mag.ing.chem.ing.

With Great Product Come Great Results








