
The pH of potable water is fundamental to ensure safe water quality.
If the pH is too low, drinking water will be corrosive to the distribution system and water pipes in homes. If it is too high, it can reduce the effectiveness of disinfectants.
The pH of water also influences aesthetic or cosmetic properties including taste, odor, and clarity. Most public water operations maintain pH between 6.5 and 8.5.
The pH of drinking water is a vital measurement. If the pH is too low, or acidic, the water will be corrosive to the distribution system and water pipes in homes. The pH of water also influences other properties including taste, odor, clarity, and efficiency of disinfection.
Most drinking water plants use surface water (lakes, rivers, and streams) or groundwater as their point source. Surface water is typically lower in mineral content, which results in lower
EC/TDS readings. Groundwater that has percolated through limestone, dolomite, or gypsum will have a relatively higher mineral content. Depending on location, there are sources of groundwater that can be very low in mineral content.
Measuring the pH of water that is low in minerals can be difficult. The lower the mineral content the less conductive the water will be. Low conductivity water presents a challenge since the pH meter is an electrochemical system that relies on the solution being measured to be conductive.
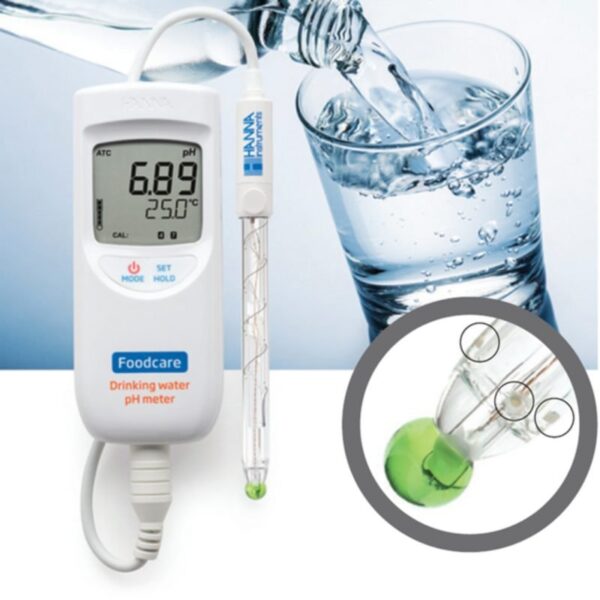
HI99192 is a portable, lightweight pH meter that is supplied with a FC2153 pH electrode designed specifically for measuring the pH of potable waters.
The pair are ideal for on-site spot checks of drinking water.
The HI99192 together with the FC2153 pH electrode solves all the problems found with standard pH systems. This specialized electrode offers numerous features that improve pH testing in drinking water. The spherical pH bulb features a low resistance pH glass that responds quickly to the sample (even at cold temperatures). It also has a refillable single junction Ag/AgCl reference that is used with a KCl electrolyte and has three ceramic junctions to ensure continuity and provide quick and reproducible measurements (even in low ionic strength waters).
The HI99192 uses the FC2153 amplified pH electrode. The FC2153 has three ceramic junctions in the outer reference cell that allows for pH measurement in low conductivity solutions.


- Simultaneous pH and temperature measurements on a large
dual-line LCD display
- User-friendly two button design
- Application specific probe
- Durable IP67 waterproof casing
- Watertight probe connection
- Probe condition indicator
- Automatic pH calibration at one or two points within two memorized
buffer sets (standard or NIST)
- On-screen calibration tags
- mV measurement for electrode check
- Selectable temperature unit (°C or °F)
- Auto-off function
- Battery life indication and low battery detection
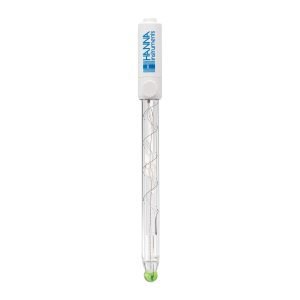

FC2153 Amplified pH Electrode
- Built-in temperature sensor
- For automatic compensation of temperature variations
- Refillable pH electrode
- Amplified electrode
- For fast, stable response that is immune to electrical noise due to humidity
- Triple ceramic junction design
The HI99192 drinking water pH meter uses the glass body FC2153 amplified pH electrode.
The amplified electrode provides a fast stable response that is immune to electrical noise due to humidity. The electrode contains an internal temperature probe to allow for automatic compensation for any variances in temperature. The electrolyte solution in the electrode is refillable. An integral part of any pH electrode is the reference junction. The reference junction allows for the flow of ions located in the reference cell into the sample being measured. The ions provide for an electrical connection between the reference electrode and the indicating electrode.


A standard pH electrode will use a single ceramic junction that allows for 15 to 20 μL/hour of electrolyte to flow. The FC2153 has three ceramic junctions providing for 40 to 50 μL/hour of electrolyte to flow.
This increased flow provides a greater continuity between the reference electrode and the indicating electrode, making it suitable for water with low ionic strength.
To optimize the flow from the electrode, the refill cap should be unscrewed; this allows for positive head pressure to be created, allowing for the electrolyte to flow more easily into the sample.


