The water has it’s own, very logical cycle. Water(l) evaporates from oceans and rivers into water vapor, condenses to form clouds, and precipitates back to Earth in the form of rain and snow filling back oceans, lakes, etc.

It all makes a perfect sense, but, did you ever wonder where it all started?

Flowing water actually had a very long journey, has not yet been totally explained. There are several possible theories at the moment, but the whole process of collecting H+ s probably started immediately after Big Bang, but 02-s are the enigma.
It probably arrived much later from outer space, using asteroids as transport vessels.
In any case, life that we know today took billions of years to form and lead to forming WATER as we know today:
2H2(g)+O2(g)→2H2O(l)

When we take a look at a globe , our planet looks like it has a lot of water. Statistically, 71% of the Earth is covered in water.
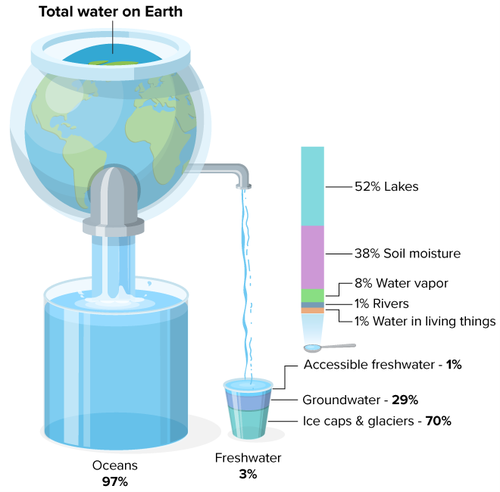
Unfortunately, we can’t use most of that as drinking water. Over 97% of the Earth’s water is salt water in oceans and seas. Another 2% is frozen in icecaps and glaciers. That leaves less than 1% of the Earth’s water for everyone to drink. As the Earth’s population grows, and many countries are further developed, fresh water is becoming more and more limited.
Distribution of water on Earth:
-
Ocean Water: 97.2%
-
Icecaps and Glaciers: 2%
-
Water in the Atmosphere: 0.001%
-
Water in Salt Lakes & Inland Seas: 0.008%
-
Groundwater: 0.62%
-
Fresh Water Lakes: 0.09%
-
Rivers: 0.0001%

Climate change, increasing water scarcity, population growth, demographic changes and urbanization already pose challenges for water supply systems. By 2025, half of the world’s population will be living in water-stressed areas. Re-use of wastewater, to recover water, nutrients, or energy, is becoming an important strategy.
Increasingly countries are using wastewater for irrigation – in developing countries this represents 7% of irrigated land. While this practice if done inappropriately poses health risks, safe management of wastewater can yield multiple benefits, including increased food production.
Options for water sources used for drinking water and irrigation will continue to evolve, with an increasing reliance on groundwater and alternative sources, including wastewater. Climate change will lead to greater fluctuations in harvested rainwater. Management of all water resources will need to be improved to ensure provision and quality.
For drinking water the most important is to accurately monitor the characteristics and provide proper quality control.
Special attention should be payed to the method of obtaining, purification, sampling, and monitoring of the chemical composition with the maximum permissible values of individual elements.
How do you know drinking water is safe?
There are prescribed minimum requirements for parametric values used to assess the quality of water intended for human consumption.
Analysis of drinking water
The analysis of drinking water includes the following determinations:
- Organoleptic and physico-chemical properties of water:
- color, temperature, odor and taste
- turbidity, pH and electrical conductivity;
- Chemical properties of water: hardness, calcium, magnesium, potassium, lithium, sodium, ammonia, fluoride, chlorite, bromate, chloride, nitrite, chlorate, bromate, nitrate, phosphate, sulphate, oxidative, dissolved gases in water, fats and oils, mineral oils, free residual (chlorine) residues, detergents;
- Bacteriological properties of water: Intestinal enterococci and Escherichia coli (E. coli) which should be absent (0/100mL)
- Toxic (toxic) substances: aluminum, arsenic, beryllium, cyanides, cadmium, chromium, nickel, lead, pesticides, selenium, vanadium, mercury, hydrogen sulfide, copper, zinc and others.
Turbidity
Turbidity is one of the most important parameters used to determine the quality of drinking water. Once considered as a mostly aesthetic characteristic of drinking water, significant evidence exists that controlling turbidity is a competent safeguard against pathogens. In natural water, turbidity measurements are taken to gauge general water quality and its compatibility in applications involving aquatic organisms. The monitoring and treatment or wastewater was once solely based on the control of turbidity.
Turbidity of water is an optical property that causes light to be scattered and absorbed, rather than transmitted. The scattering of light that passes through a liquid is primarily caused by the suspended solids present. The higher the turbidity, the greater the amount of scattered light. Even a very pure liquid will scatter light to a certain degree, as no solution will have zero turbidity.
HI88713-02
ISO 7027 Compliant Benchtop Turbidity Meter
The HI88713 is a high accuracy benchtop turbidity meter. The meter is supplied complete with AMCO-AEPA-1 primary turbidity standards used for calibration and performance verification. The HI88713 meets and exceeds the requirements of the ISO 7027 Method for turbidimetric measurements.
- Compliant to ISO 7027 method
- Ratio and non-ratio turbidity modes
- USB for data transfer


HI801
Spectrophotometer Iris
IRIS portable spectrophotometer is unlike any of the products we have created in the past. It is different from our photometers as it allows for measurement in the spectrum of all wavelengths of visible light and not just pre–specified wavelengths. Spectrophotometers work by isolating light at specific wavelengths from white light. This compact meter incorporates a number of features that facilitate both fantastic performance and exceptional usability.
- Advanced split beam optical system
- Rechargeable li-ion battery
- User customizable methods
Some of the parameters include alkalinity, calcium, nitrite, and phosphate which are critical to maintaining a healthy system. It also contains parameters specific to either a marine or freshwater environment. You can also measure dissolved oxygen, ammonia, calcium, free chlorine, total chlorine, copper, nitrate which are important parameters for aquaculture and all water living organisms.
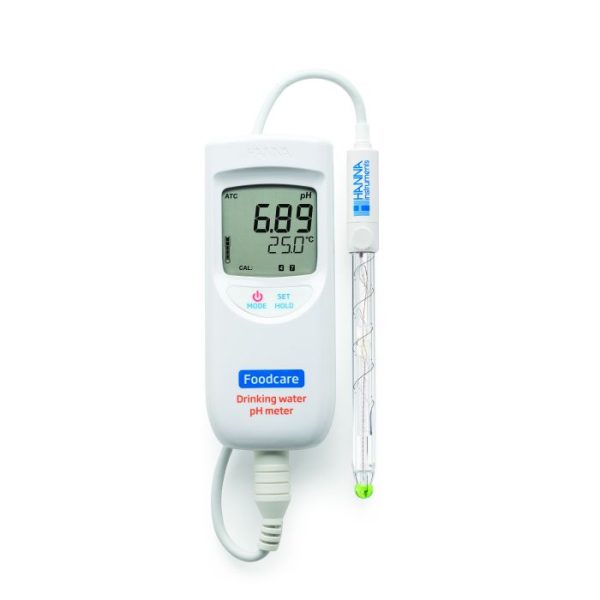
HI99192
Drinking Water pH Portable Meter
Designed to bring simplicity to pH testing of drinking water, the HI99192 Drinking Water pH Meter is designed for measuring the pH of potable waters.
- ±0.2 pH accuracy
- Specialized probe for drinking water.
- Comes with all the necessary solutions and batteries- everything you need to get started measuring right away.


HI98494
Portable pH/EC/DO Meter with Bluetooth
Functional and accurate, this meter is capable of testing 12 different water quality parameters using pH, EC, and optical DO sensors. Transfer data to a smart device for review or sharing with the integrated Bluetooth connection and Hanna Lab App. Device is waterproof ( meter rated IP67, probe rated IP68). It has automatic interval logging of up to 45,000 samples or log-on-demand. HI98494 is perfect for environmental and industrial professionals.
HI98194
Multiparameter pH/ORP/EC/TDS/Salinity/DO/Pressure/ Temperature Waterproof Meter
The HI98194 is a waterproof portable logging multiparameter meter that monitors up to 12 different water quality parameters including 6 measured and 6 calculated. The microprocessor based multi-sensor probe allows for the measurement of key parameters including pH, ORP, conductivity, dissolved oxygen, and temperature.
The probe transmits readings digitally to the meter, where data points can be displayed and logged. The HI98194 is supplied with all necessary accessories and packaged in a durable carrying case.


HI9829
Multiparameter pH/ISE/EC/DO/Turbidity Waterproof Meter with GPS option
The HI9829 is a waterproof portable logging multiparameter meter that monitors up to 14 different water quality parameters. The microprocessor based multi-sensor probe allows for the measurement of key parameters including pH, ORP, conductivity, dissolved oxygen, turbidity, ammonium, chloride, nitrate, and temperature. The probe transmits readings digitally with options to log data while disconnected from the meter. An optional GPS provides location tracking of measurements. The complete system is simple to setup and easy to use. The HI9829 is highly customizable and supplied with all necessary accessories, packaged in a durable carrying case.
Two probes to choose from (Basic or Logging)
The HI7609829 (basic) and HI7629829 (logging) are multiparameter probes for use with the HI9829 portable meter. It is an option to choose which probe will be supplied with the HI9829. By default the HI9829 and corresponding probe will be supplied with a pH/ORP, conductivity and dissolved oxygen sensors. Either probe can be upgraded to measure turbidity with a turbidity/conductivity sensor.

HI98198
Portable Optical DO (opdo) meter
HI98198 uses a luminescent optical method for the measurement of dissolved oxygen in water and wastewater.
This professional, waterproof meter complies with IP67 standards and measures:
- DO
- Barometric pressure
- BOD
- Temperature
The HI98198 is supplied complete with all accessories including probe, smartcap sensor with built in RFID, and a rugged carrying case.

Advantages: HI98198 Dissolved Oxygen Meter has many advantages over other Galvanic and Polarographic Dissolved Oxygen Meters. This meter uses HI764113 Rugged Optical Dissolved Oxygen Probe for Dissolved Oxygen measurement which has following benefits.
- No membranes
- No electrolytes
- No oxygen consumption
- No flow dependence or minimum flow rate
- Fast and stable readings
- Minimal maintenance
Advanced Tools for Advanced Needs
Automatic titrators from Hanna combine advanced performance with untouched affordability.

Mini Titrator for Titratable Alkalinity
Advanced Automatic Potentiometric Titrator
Author:
Nives Vinceković Budor, mag.ing.chem.ing.
Sources:
https://www.who.int/news-room/fact-sheets/detail/drinking-water
https://www.worldwildlife.org/industries/freshwater-systems
https://www.un.org/en/global-issues/water
https://extension.psu.edu/the-water-we-drink
https://data.unicef.org/topic/water-and-sanitation/drinking-water/
https://apps.who.int/iris/bitstream/handle/10665/39989/9241540249_eng.pdf;sequence=1



