Sodium can be found naturally and as an additive in food products. Most of the times it is added to food products in the form of sodium chloride (NaCl) but can also be added in other forms including sodium nitrite, sodium bicarbonate (baking soda), sodium benzoate, and monosodium glutamate (MSG). They are added to foods to enhance flavor and other organoleptic properties, act as a binder, and/or to inhibit microbial growth to extend the shelf life of a product.
It is important to note that not all salts contain sodium chloride. There are other salts, including potassium chloride (KCl) and calcium chloride (CaCl2). But generally speaking, sodium chloride is one of the most common salts added to food.
It is necessary to intake sodium because it regulates nervous and muscular systems. It is found mainly in the blood and extracellular fluids. The amount of sodium intake can have a great impact on blood pressure. There are many detrimental effects of high blood pressure including an increased risk of cardiovascular disease, congestive heart failure, and kidney disease.
Methods of Sodium Analysis in food
For measuring concentration of sodium (salt) in food, there are several different methods available. Each method has its own limitations and advantages. Considerations in determining the appropriate methods include equipment cost, accuracy desired, and experience level of the person performing the test.
The most common measurement methods for determining sodium salt content include:

Refractometry
By conducting this method, salt content of a substance is based on refractive index. The refractive index is determined by passing a light through a prism into a sample and measuring how the light bends. Refractometers determine the critical angle of a sample. The critical angle is the angle at which no light is refracted and all light is internally reflected.

Each refractometer is based on the effect of density and temperature on the refractive index for a specific measured parameter. The refractive index is converted to a measurement unit such as % salt.
Digital refractometers utilize an internal light source at a fixed wavelength. This internal light passes through a prism and into the sample and an internal light detector identifies the critical angle. Digital refractometers remove the subjectivity of determining the shadow line manually and have improved temperature compensation due to the use of pre-programmed algorithms; they can also perform measurements over wider temperature ranges at a low-moderate price investment.
Refractometers are attractive due to their low startup cost and that they use no chemical reagents. A small sample is all that is needed. This makes them ideal for quantitative use in simple solutions, such as a salt brine solution. However, this method is not specific to salt. Other substances present in the sample will alter the refractive index. These substances include fats, sugars, and minerals.
HI96821
Digital Refractometer for Measuring
Sodium Chloride in Food
Sodium Chloride in Food

The HI96821 is a rugged, portable digital refractometer designed for sodium chloride (NaCl) measurement. The HI96821 displays NaCl concentration four different ways: g/100 g, g/100 mL, specific gravity, and °Baume. The instrument’s high accuracy and simple operation gives reliable results each and every time. All readings are automatically compensated for temperature variations and displayed with a 1.5 second response time. The sealed flint glass prism and stainless steel well are easy to clean. Just wipe with a soft cloth in preparation for the next sample.
Electric conductivity (EC)
Table salt dissociates as two ions in solution: sodium and chloride. Since ions are charged particles, electricity is conducted more easily. As a result, an electrical conductivity (EC) meter can be used to estimate the amount of salt dissolved in solution. Once an EC measurement is obtained, a conversion factor specific to salt must be applied in order to get the amount of salt in a solution. Many meters have this calculation built-in.
There are two main types of probes used in measuring conductivity in foods: two electrode probes and four-ring probes.
But, you should remember that EC is only able to tell you the concnetration of ions in solutions, but not what those ions are. If the sample is more complexed, some other methodes (such as titraton) would be more suitable.
Conductivity represents an attractive option for a QC setting for its ease of use and affordability. However, conductivity probes measure conductivity, not specific salts. This means that any ions (e.g. calcium, magnesium, etc.) will interfere with measurements. Thus, conductivity measurements only estimate the salt content rather than provide an exact value.

HI2003
Dedicated EC Meter and TDS/Salinity
Meter edge®
Meter edge®
edge® groundbreaking design is the culmination of Hanna’s vision, design capabilities, integrated production and world-class R&D. edge® is a single meter that can measure Conductivity, TDS and Salinity and is incredibly easy to use.
- edge® EC/TDS/Salinity utilizes four ring conductivity probe technology that allows the user to measure samples from very low conductivity to very high conductivity.
- edge® features a capacitive touch keypad that gives a distinctive, modern look. The keypad is sensitive enough to be used with laboratory gloves and has a fast response.
- edge® features a 5.5” LCD display that you can clearly view from over 5 meters away. The large display and it’s wide 150° viewing angle provide one of the easiest to read LCD’s in the industry.
Ion-Selective Electrode (ISE)
The third method for determining salt content in food is with the use of an ion-selective electrode. This is often referred to as an ISE.
An ISE is a chemical sensor used to determine the concentration of a specific ion in a solution. In sodium ISEs, the tip is a sodium-sensitive glass bulb. Like a pH electrode, ISEs obey the Nernstian response, which allows us to correlate a millivolt (mV) reading to a concentration value.
The ISE must be calibrated daily in order to ensure accurate measurements. Calibration standards should bracket the expected concentration of the sodium content of the food. For example, one calibration standard should have a higher concentration than the expected value, and the other a lower concentration.
Ionic strength adjuster (ISA) must also be added in a fixed ratio to both calibration standards and samples for accurate readings. Electrode response is affected by ion concentration and ion activity. The ISA standardizes ion activity between calibration standards and samples, ensuring that changes in the electrode response are based on changes in ion concentration. Once calibration is complete, measurements of liquid or solid samples can be performed. Solid samples require pre-treatment in order to make a slurry that can then be measured with the probe.
Sodium ISEs are specific to sodium measurement and are prone to little interference. The startup cost to measure sodium with an ISE is moderate. A pH meter with an ISE concentration readout or a dedicated sodium ISE meter is needed along with the ISE, ISA and calibration standards.
FC300B
Sodium Combination
Ion Selective Electrode (ISE)
The FC300B is a glass combination ion selective electrode (ISE) for the determination of sodium (Na+) ions in solution.
The selective glass membrane produces a potential change due to the exchange of sodium ions on the surface of the membrane and the sample. The internal sensing elements are housed within a sturdy glass body.
The FC300B is ideal for a variety of applications in laboratories, food and beverage production, and water quality analysis.
- Refillable Glass Body
- Single Ceramic Junction
- Detection from 0.23 to 22,990 mg/L Na+
Titration
Titration is the most common method of salt analysis by a food manufacturer with an in-house laboratory.
A titration method is referenced by organizations including AOAC for a variety of food products including cheeses, meats, and vegetables. A titration is a procedure where a solution of a known concentration (titrant) is used to determine the concentration of an unknown solution (analyte).
Results are calculated based on the amount of titrant used to reach an endpoint. An endpoint can correspond to a color change with the use of a chemical indicator, or detection with a potentiometric sensor, such as a chloride or silver ISE.

Automated Titration:
Potentiometric Method
Titration with silver nitrate can be automated with a potentiometric titration system. A titration system for salt analysis is equipped with an ISE or silver billet electrode. ISEs are sensitive to the concentration of either chloride or sliver ions. Silver billet electrodes provide a lower maintenance option that meets industry standards. Both electrodes are used to monitor the solution for a change in the mV potential as a result of pertinent ions being either consumed or in excess.
Potentiometric titration systems automatically control titrant dosing and endpoint detection. Automatic endpoint detection increases titration precision and accuracy by eliminating the human subjectivity that is associated with a titration using a visual indicator. Instead of monitoring for a color, the titrator determines the endpoint by measuring changes in mV potential. It is important to note that there is no calibration of an ISE or of a silver billet with potentiometric titration, since it is an abrupt mV change that is important.
The automated dosing system of a potentiometric titrator also increases precision due to the ability to dispense and measure finite amounts of titrant.
Automatic titrators will also perform all of the necessary calculations and display the results in the concentration units desired. The other benefits of an automatic titration system include the ability to generate reports for traceability and the option to perform other titrations including acidity.
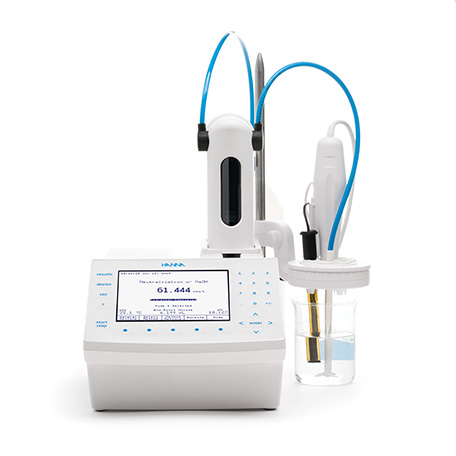
HI931-02
Automatic Potentiometric Titrator
The HI931 Automatic Titrator is the answer to your dedicated titration needs. Fully customizable, the HI931 delivers accurate results and intuitive user experience, all in a compact package. Titrate for a variety of measurements at the push of a button including acids, bases, redox, and selective ions. No additional programming upgrades to purchase. The only things you need to start using the HI931 are a sensor and titrant.
- Small footprint so you can fully optimize your benchtop.
- Unmatched 40,000-step dosing pump for small volumes of titrant to help you achieve a very precise endpoint for greater consistency.
- Flexibility to store up to 100 methods.
Author:
Tajana Mokrović, mag.nutr.
Tajana Mokrović, mag.nutr.