While edible oils (such as coconut oil, olive oil, sunflower oil…) share many qualities, consumers may choose a particular oil based on its cost, unique health benefits, and characteristic ability to enhance meal flavors. Each oil has its own shelf life. It depends on the composition of the oil itself, its packaging and storage and handling after opening. Over time, edible oils may degrade and spoil. The primary cause of edible oil degradation is oxidation. As oil oxidation occurs, flavors and odors can change, resulting in a product that is undesirable for consumers. The primary products of oxidation are peroxides, hydroperoxides, and free radicals. They act as catalysts for the whole reaction and accelerate it. Secondary products are aldehydes, ketones, alcohols, fatty acids, oxy fatty acids, epoxides that cause rancidity of the product. Peroxides easily lose one oxygen atom and turn into ordinary oxides and are therefore strong oxidizing agents.


This oxidation reaction is more likely to occur under certain conditions, including exposure to light, the presence of metal ions, the introduction of oxygen, or when storage temperatures are not maintained. In order to determine the oil quality and the onset of oxidation, peroxide value is determined. Peroxide value is defined as the amount of peroxide oxygen per kilogram of oil, which is reported in units of milliequivalents or meq.
The peroxide value tells us a lot about the quality of the oil. Peroxide number indicates the amount of hydroperoxide and peroxides present in the oil. Oils with a peroxide value ranging from 1 to 3 mmol O2 / kg are considered fresh and of good quality. Oils whose peroxide value does not exceed 10 mmol O2 / kg are considered suitable for human consumption.The peroxide number is considered an indicator of the initial phase of oil oxidation because the resulting hydroperoxides are extremely unstable and decompose very quickly in the so-called secondary oxidation products.
For measure peroxide values in oil we recommend Portable Photometer for Determination of Peroxide Value in Oils – HI83730


The HI83730 Portable Photometer for the Determination of Peroxide Value in Oils combines accuracy and ease of use in an ergonomic, portable design. A user can accurately determine the peroxide value of olive oil within a 0.0 to 25.0 meq O2/kg range using the HI83730-20 ready made reagents.
For a determinating peroxide number by Reference Method AOCS Cd 8b-90 or Reference Method AOAC 965.33 use Hanna Instruments HI931 Automatic Potentiometric Titrator.
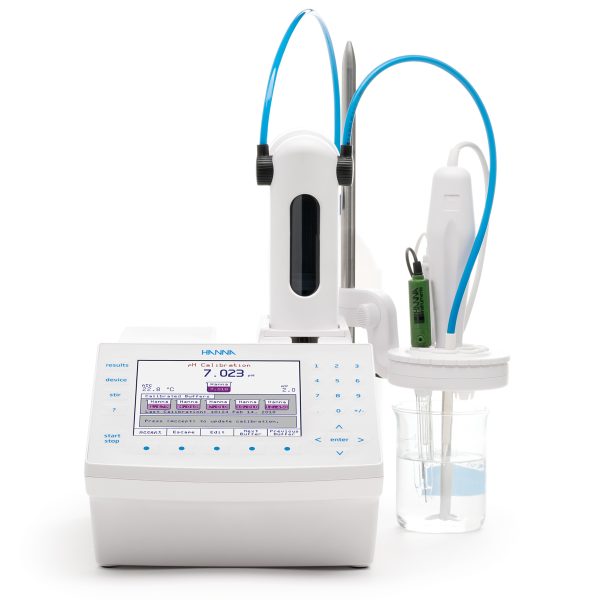

The HI931 Automatic Titrator is the answer to your dedicated titration needs. Fully customizable, the HI931 delivers accurate results and intuitive user experience, all in a compact package. Titrate for a variety of measurements at the push of a button including acids, bases, redox, and selective ions. With no additional programming upgrades to purchase, you can start measuring right away. This titrator has the ability, among many other methods, to titrate the value of the peroxide number and for this measurement there are two standardized methods.
Method for the determination of peroxide value (PV) in refined and unrefined edible oils, following the AOAC 965.33 to a mV endpoint. The results are expressed in meq/kg PV (Peroxide Value).
Method for the determination of peroxide value (PV) in refined and unrefined edible oils, following the AOCS Cd 8b-90 to a mV endpoint. The results are expressed in meq/kg PV (Peroxide Value).
Contact us for more information about quality of your oil.
Author: Tajana Mokrović, mag.nutr.



