Volatile acidity (VA) is one of the most important parameters determined during production and storage of wine.
It is important due to the chemical and microbiological stability of wine, preserving its specific taste and color, and it is linked to wine quality [1].
Volatile acids in wine are formed as secondary products of alcoholic fermentation or in the process of wine spoilage. They affect the sensory properties of wine and are indicators of biological (in) stability of the wine.
Under certain conditions (such as heating) it evaporates from the wine. The total share of volatile acids is expressed as acetic acid because it accounts for 99% of all volatile acids in wine. [2].
For measuring volatile acid in wine there are several methods:
using a titrator
with spectrophotometer
The origin of acetic acid in wine
- It goes from grapes to wine only if it is spoiled or damaged (mold, insects …)
- Secondary product of alcoholic fermentation:
a) dismutation of acetaldehyde
acetaldehyde + H2O → acetic acid + EtOH
b) during fermentation:
yeasts, temperature, sugar content, air access - Biodegradation of citric acid
- After alcoholic fermentation – during storage of wine:
a) oxidation of ethanol with oxygen from the air
ethanol + O2 → acetaldehyde + H2O
acetaldehyde — O2/H2O → acetic acid
b) larger quantities occur as a result of some spoilage caused by bacteria (wine flower…)


Healthy wine contains 0.3-0.6 g / L; less often up to 1 g / L.
Rotten wine contains 2-3 g / L.
According to the Ordinance on wine, the maximum is allowed [3]:
- white, rose, black cherry with a content of up to 10% vol alcohol: 1.1 g / L acetic acid
- wine with a label of controlled origin: 1 g / L acetic acid
- wine with more than 10% vol alcohol: for each vol. % alcohol is allowed another + 0.06 g / L acetic acid
Other volatile acids in wine: propionic acid, butyric acid, valeric acid, then caproic, caprylic, capric, pelargonium, lauric, etc.
For measuring volatile acid in wine there are several analytical methods
1. Measurement of volatile acids using a titrator
This method requires the steam distillation (using a modified-Markham or Cash still) of a wine sample that has first had sulfur dioxide removed by oxidation with hydrogen peroxide.
The distillate is titrated with sodium hydroxide to end point pH 8,20 indicated with pH electrode and the acidity calculated and expressed as acetic acid equivalents.
- Needed equipment: Modified-Markham or Cash still, automatic titrator, pH electrode, glossary
- Reagents: Standardised sodium hydroxide solution, calibration solutions for pH electrode, hydrogen peroxide solution
- Services: Electricity, water supply, sink, wash up area, natural gas supply
- Space required: Bench space
Pros: saves time, reduces and rationalizes the consumption of chemicals, more accurate results, more wine parameters can be measured on titrator
Cons: initial cost of equipment, sample preparation
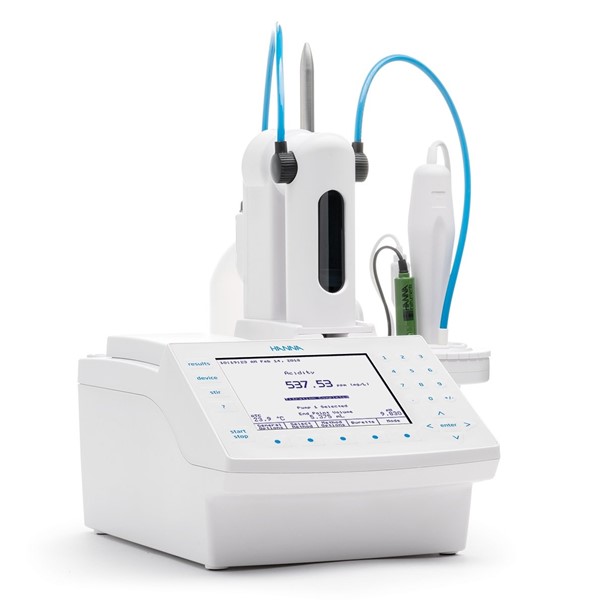
HI931
Automatic Potentiometric Titrator
The HI931 Automatic Titrator is the answer to your dedicated titration needs.
Fully customizable, the HI931 delivers accurate results and intuitive user experience, all in a compact package.
Titrate for a variety of measurements at the push of a button including acids, bases, redox, and selective ions.
No additional programming upgrades to purchase. The only things you need to start using the HI931 are a sensor and titrant.
- Small footprint so you can fully optimize your benchtop.
- Unmatched 40,000-step dosing pump for small volumes of titrant to help you achieve a very precise endpoint for greater consistency.
- Flexibility to store up to 100 methods.
2. Measurement of volatile acids with spectrophotometer
Since volatile acidity is mainly comprised of acetic acid (99%), it is sometimes useful to measure the concentration of acetic acid alone.
The conversion of acetic acid by aldehyde dehydrogenase can be monitored directly by measuring the absorbance (340 nm) resulting from the generation of a by-product of the reaction (NADH).
The test is quite straightforward to conduct and requires only sample dilution. Kits for this assay are commercially available.
- Needed equipment: UV spectrophotometer and cuvettes, autopipettes
- Calibration: Made-up and kit-supplied standard solutions of glucose in water
- Services: Electricity, wash up area
- Space required: Bench space depending on spectrophotometer footprint
Pros: simple sample preparation, saves time, more accurate results, the small footprint of instrument
Cons: cost of assay kits, need to calculate the result, cost of instrumentation [4,5,6]
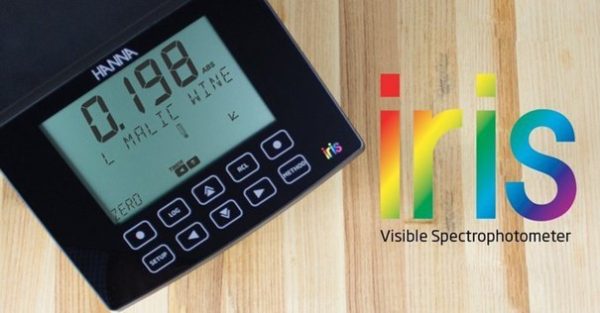
HI801
The HI801 Iris is a sleek and intuitive spectrophotometer that allows for measurement of all wavelengths of visible light.
Customize your methods, take a wide range of measurements, and feel confident in your testing accuracy with iris.
- Iris features precise wavelength selection between 340 nm to 900 nm for complete method compliance and accuracy that is necessary in industries like professional laboratories, water treatment facilities, wineries, and more.
- Results are consistent and accurate regardless of throughput with the high quality and uniquely designed optics system.
- Customization options include multiple cuvette shapes and sizes, custom calibration curves, and methods.
Author: Tajana Mokrović
mag.nutr.
Have questions?
Contact a Hanna Technical Specialist at info@hannaservice.eu or using our contact form.
SOURCES:
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dobourdieu, D. The Chemistry of Wine. In Handbook of Enology, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Volume 2, pp. 3–10.
- Zoecklien, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production, 1st ed.; Springer: Boston, MA, USA, 1999; pp. 192–198.
- https://narodne-novine.nn.hr/clanci/sluzbeni/2022_07_81_1183.html
- Amerine, M.A.; Ough, C.S. (1980) Methods for analysis of musts and wines. New York Wiley-Interscience.
- Coulter, A. 2020. Ask the AWRI: Discrepancies in analytical results for volatile acidity Aust. N.Z. Grapegrower Winemaker (674): p.64.
- Coulter, A. 2018. Ask the AWRI: Volatile acidity. Aust. N.Z. Grapegrower Winemaker (648): p. 16.