Soil is one of the most important natural resources and represents the basic base for the production of organic matter. Soil is a natural substrate from which plants draw essential elements that are necessary for their proper growth and development.
Land is a limited natural good that is destructible, slowly formed and quickly destroyed in the process of improper use. It is the basis of agricultural production and a condition for the survival of the living world on our planet.

The productive ability of land is a factor that determines the productivity of agricultural production and implies the ability of plants to be supplied with water and essential minerals through the root system.
Soil fertility is another important factor of any agricultural land. Fertility is a dynamic state of various physical, chemical and biological properties and processes in the soil, thanks to which a different degree of life of plants, animals and even humans is possible.
By using the land in intensive plant production, the balance of certain factors of soil creation and damage is often disturbed.
Man’s activity, most often agricultural production, can increase or decrease the fertility of the land. Factors that endanger the fertility of the soil can be: reduction of stocks of organic matter, compaction of the soil, deterioration of the structure and deterioration of the water-air and heat regime, reduction of biological activity, i.e. disorders in the number and presence of different groups of beneficial microorganisms, pollution by pesticide residues, pollution by heavy metals, radionuclides, salinization, alkalization and acidification of soil, erosion, temporary and permanent soil losses due to changes in agricultural land use and more.

Agrochemical fertility of soil implies chemical properties of soil that are determined by the content of macro and microelements (N, P, K, Ca, Mg, S, Fe, Zn, Mn, Cu, Co, Mo, B, Se, Si, Na, Cl and others ) and the absence of soluble forms of harmful elements (some heavy metals, radionuclides, etc.).
Heavy metals in the soil can be of natural and anthropogenic origin.
In nature, heavy metals reach the soil by the natural decomposition of rocks and minerals on which the soil is formed, which also contain heavy metals. The most common are Cu, Zn, Ni, Pb, Al, Cr.
The natural content of heavy metals in the soil is usually low, with the exception of land near mines and metal deposits.
In some soils there is a higher concentration of heavy metals than their concentration in the rocks and minerals on which that soil was formed.
The causes of the increase in heavy metals in such lands can arise as a consequence of industrial plants for metal processing, traffic and the use of fossil fuels.
Due to these human activities, metals are released into the air, and in the form of rain, gases and soot they reach the surface of the earth. Exhaust gases from cars pollute the ground with metals in the immediate vicinity of roads (up to 100m).
In the process of growing plants, various agrotechnical measures are applied in order to improve the yield and control pests.

For this purpose, numerous means for plant nutrition and protection, mineral fertilizers and pesticides are used. In addition to their beneficial effects on plants, these products can also be harmful if used sparingly. In this case, soil and plants can be contaminated with heavy metals.
Uncontrolled use of industrial and municipal wastewater for irrigation can cause contamination of the soil with heavy metals. The use of compost from city garbage and sewage sludge also leads to contamination of the soil with heavy metals.
MACRO AND MICROELEMENT

MACROELEMENTS
N (Nitrogen)
Nitrogen is an element necessary for vegetative and generative growth and development of plants.
Nitrogen deficiency causes a decrease in plant growth, yellowing of leaves, reduction of fruits and reduction of plant resistance to diseases. Excess nitrogen causes excessive growth of plant vegetative organs, while generative growth decreases, fruit ripening is slower and plant susceptibility to disease increases.
P (Phosphorus)
Phosphorus is an element that is necessary for the growth of generative organs, plant growth, cell division, better rooting of plants, development of seeds and fruits and fruit maturation. Phosphorus deficiency slows down plant growth as well as the formation of leaves and flowers. Excess phosphorus rarely occurs, and if it does, it causes reduced plant growth, and the leaves get dark spots.
K (Potassium)
Potassium is important for plant metabolism. It affects the absorption and transport of all nutrients and water, the regulation of the pH value of cell juice, the regulation of osmotic pressure and the growth of young tissue. Increases resistance to disease. Potassium deficiency in the oldest leaves of the plant on the edges causes chlorotic and necrotic spots and twisting of the leaves. The high N: K ratio in the soil adversely affects the yield and fruit quality.
Ca (Calcium)
Calcium is an element of great importance on the structure of cell membranes.
If it is absent, the cells burst. Calcium affects cell division, root growth and elongation, plant resistance to disease. Calcium deficiency is observed in the most distant parts of the plant and in young tissue (top of roots, edges of leaves, fruits).
Mg (Magnesium)
Magnesium is an important ingredient in chlorophyll and physiological processes in plants. In the absence of magnesium, the process of photosynthesis and the decomposition of chlorophyll is stopped.
The consequences of magnesium deficiency can be seen on the oldest leaves of plants in the form of interventional chlorosis, when the nerves remain green, and the surface between them turns yellow.
S (Sulfur)
Sulfur is part of proteins, enzymes, coenzymes and amino acids. The lack of sulfur is seen on the young leaves, since sulfur is immobile in the plant.
The plant lags behind in growth, the leaves begin to turn yellow, and the stems become brittle.
MICROELEMENTS
The group of microelements includes:
Ni, B, Mn, Zn, Mo, Cu, Si, Cr, J, Se, Fe, Na, Sr, Co and Cl.
Fe (Iron)
Iron is an important element in the process of photosynthesis. Iron increases resistance to drought and diseases, and regulates the synthesis of vitamins in fruits.
Iron deficiency occurs on alkaline soils, and manifests as chlorosis between the leaf nerves. If the defect is greater, the leaves turn white or get a fried appearance.
B (Boron)
Pine enables the development of flowers, pollen fertility, fruit formation and proper fruit development. The deficiency causes drying of the lateral shoots of the fruit and reduction of the yield. The excess manifests itself as chlorosis on the leaves.
Mn (Manganese)
Manganese is an important trace element because it is a special enzyme activator. It acts as a biocatalyst in the formation of chlorophyll and improves the formation of starch and sugar in plants. Manganese deficiency may occur in alkaline soils. It manifests itself on the leaves in the form of chlorosis.
Zn (Zinc)
Zinc has multiple effects, and the most important is in the formation of chlorophyll. Zinc prevents the accumulation of excess acids in the leaves and participates in the respiration of cells. The deficiency is observed on the lateral shoots of plants, and with a greater deficiency, a reduction in yield can occur.
Mo (Molybdenum)
Molybdenum is part of the chloroplast of plants and is an important factor in photosynthesis.
It regulates the transport of iron through the plant and contributes to the absorption of nitrogen. The deficiency causes the older leaves to bend in the middle.
Cu (Copper)
Copper directly and indirectly affects many physiological processes in the plant, increases yield, increases fruit quality and accelerates ripening. In the absence of copper, there is less fertilization, the young plants wither, and the leaves turn ashy and dry.
Influence of heavy metals on plants
Some heavy metals in small quantities are necessary for the proper growth and development of plants. These are Fe, Zn, Cu, Se and Co. Some heavy metals are toxic Hg, Pb, As, Ni, Cr (VI) and Cd.
Plants play an important role in the circulation of metals in nature. Heavy metals enter the food chain through plants. Heavy metals affect all physiological and biochemical processes of plants. Plants have elaborate mechanisms for detoxification of heavy metals: binding of metal to the cell wall and excretion from the root, restriction of movement towards the root, active pumping, repair and protection of plasma membranes and chelation of metals.
The adverse effect of heavy metals on plants is first noticed as a slow growth of the plant, and then the appearance of chlorosis and necrosis. Changes are observed on the oldest leaves of the plant, and later on other leaves. Leaf extinction occurs, and high concentrations can lead to plant extinction.

Experimental data show that heavy metals affect the process of photosynthesis. Plants adopt them through the root system in the form of ions and / or organic chelate complexes.
When heavy metals, such as Ag, Hg or Cu, come into contact with the plant’s root, they affect the selectivity of the parts of the cells responsible for transporting nutrients. In this way, ion carriers lose their function. The intensity of uptake and accumulation of heavy enamels in root cells also depends on the presence of other ions in the nutrient substrate. Pb, Ni and Cd inhibit the uptake of Ca, Mg, Fe, Zn, Mn and Cu and prevent their transport from the roots to the aboveground parts of plants. In that way, they adversely affect the distribution of nutrients in plants. With a large excess of Ni and Cd in the nutrient medium, the metabolism of Mg, Fe, Mn and Zn is completely stopped. Chlorosis and necrosis appear in the leaves, and the root has a higher concentration of these elements. Excess Ni adversely affects the uptake of Fe and the uptake of Co and thus reduces the incorporation of Co into vitamin B12. Cd acts on the selectivity of root cell membranes and the activity of enzymes present in the membranes. This affects the growth of root cells, the uptake and transport of water, and causes an increase in the concentration of phenolic compounds.
At very low concentrations, Pb affects the uptake of Ca, K, P, Mg, Fe, Cu, Zn and Mn.
Excess Cu differently affects the content and transport of other elements. At the root, it reduces the concentration of Ca and Fe, and to a lesser extent to the adoption of K.
A great toxic effect on plants occurs when the presence of several toxic metals is combined.
Cd (Cadmium)
High concentrations of Cd in plants affect iron metabolism, cause chlorosis and thus affect the process of photosynthesis.
Pb (Lead)
Lead in high concentrations slows down the process of root elongation, plant growth, slows down the process of photosynthesis and affects the structure of plants.
Hg (Mercury)
Mercury disrupts the structure of bio membranes and alters enzyme activity. In this way, the metabolism is disturbed, which leads to reduced plant growth.
Cr (Chrome)
Chromium in higher concentrations has a toxic effect on plants. It causes chlorosis and reduces growth, as well as seed germination.
Ni (Nickel)
Excess nickel causes chlorosis. It adversely affects the mobility and translocation of iron and its absorption.
Cu (Copper)
Copper can have a toxic effect if its concentration in the soil reaches 2540 mg / kg if the pH value of the soil is below 5.5. High concentrations of copper occur in acidic soils.
Zn (Zinc)
High concentrations of zinc in plants occur on acidic soils, such as in the vicinity of zinc mines and smelters. In these cases, the plants have reduced growth, reduced root system, formation of small leaves and necrosis of leaves.
As (Arsenic)
High concentrations of arsenic and its toxicity occur on acidic soils, especially if the pH of the soil is less than 5. Toxicity is higher on sandy soils than on denser soils.
Se (Selenium)
High concentrations of selenium inhibit plant growth and cause chlorosis. Selenium accumulates most in the seed during plant growth. In this case, the smell of garlic appears in plants, which indicates a high concentration of selenium.
Permitted concentrations of heavy metals in the soil
Modern agricultural production requires high and stable yields of good quality, with minimal investment of materials, energy and labor, and protection of the environment from harmful effects and pollution.
In order to achieve this, it is necessary to know all the factors that can affect soil fertility, and thus higher yields.
The soil needs to be provided with an ideal ratio of nutrients. If there are too many nutrients, groundwater and surface water, air and soil are polluted.
In order to properly manage nutrients, it is necessary to perform an analysis of soil, fertilizers, and it is necessary to know what the needs of certain plants are for nutrients. In this way, a better yield is achieved, and environmental pollution is reduced. In this way, savings are made when buying mineral fertilizers.
The concentration of heavy metals in the soil is controlled by the limit values of maximum permissible concentrations (MAC). In most countries in Europe, it is legally binding, while in some it is non-binding recommendations.
The limit that determines the maximum permitted amount of a harmful substance in the unit volume of the observed medium represents the maximum permissible concentration (or MPC). Lately, this quantity has been called the immission limit value (GVI).
The maximum permissible concentrations refer to the total content of heavy metals.
Hazardous substances are usually: Cd, Pb, Hg, As, Cr, Ni and F, and harmful substances are Cu, Zn and B.
A special rulebook prescribes the maximum permitted quantities of hazardous and harmful substances in soil and irrigation water that can damage or change the productive capacity of agricultural land and the quality of irrigation water, which come from discharge from factories, landfills, improper use of mineral fertilizers and pesticides. plants.
The maximum permitted quantities of hazardous and harmful substances are:
Chemical element | MPQ at soil mg/kg of soil | MPQ in the water mg/l of water | ||
1 | Cadmium | Cd | up to 3 | up to 0,01 |
2 | Lead | Pb | up to 100 | up to 0,1 |
3 | Mercury | Hg | up to 2 | up to 0,001 |
4 | Arsenic | As | up to 25 | up to 0,05 |
5 | Chrome | Cr | up to 100 | up to 0,5 |
6 | Nickel | Ni | up to 50 | up to 0,1 |
7 | Fluoride | F | up to 300 | up to 1,5 |
8 | Copper | Cu | up to 100 | up to 0,1 |
9 | Zinc | Zn | up to 300 | up to 1,0 |
10 | Boron | B | up to 50 | up to 1,0 |
Hanna Instruments portfolio includes instruments with which you can perform soil analyzes for the content of macro and micro elements.
-Multiparameter photometer HI83325
-Suction Lysimeter HI83900
-Iris HI801 Spectrophotometer
–Hanna Checker HC Series
Multiparameter photometer HI83325
Photometer HI83325 ensures accurate and repeatable photometric readings every time. Key parameters are: Ammonia, Calcium, Magnesium, Nitrate, Phosphorus, Potassium, Sulphate and pH.


Advanced optical system – Unparalleled performance from a benchtop photometer. Consistent and thorough monitoring of plant nutrients is essential to maintaining healthy growth and reproduction. This is easy with the HI-83325, a comprehensive way to monitor vital plant nutrients such as potassium, calcium and magnesium. Required in large quantities, potassium plays a vital role in water uptake and enzyme regulation. Calcium helps to strengthen plant cell walls protecting against heat stress while magnesium helps build a strong immune system.
A digital pH electrode input allows the user to measure pH by a traditional glass electrode.
The HI-83325 offers an absorbance measuring mode that allows for CAL Check standards to be used to validate the performance of the system. The absorbance mode allows the user to select one of the three wavelengths of light (420 nm, 466 nm, and 525 nm) to measure and plot their own concentration versus absorbance mode. This is useful for users with their own chemical method and for educators to teach the concept of absorbance by using the Beer-Lambert Law.
Two USB ports are provided for transferring data to a flash drive or computer and to use as a power source for the meter. For added convenience and portability the meter can also operate on an internal 3.7 VDC Lithium-polymer rechargeable battery.
Key features:
- Advanced optical system with brighter, long-lasting LED light source
- Backlit 128 x 64 Pixel Graphic Liquid Crystal Display
- Built-in Reaction Timer for Photometric Measurements
- Absorbance mode – concentration versus absorbance
- Units of Measurement plus chemical form displayed
- Result conversion at the touch of a button
- Cuvette cover
- pH and temperature measurement with a single probe
- Good Laboratory Practice (GLP) – track calibration information including date, time, buffers used, offset and slope for traceability
- CAL Check alerts user to potential problems during the calibration process
- Data Logging – Up to 1000 photometric and pH readings can be stored.
- Logged readings can be quickly and easily transferred to a flash drive or to a PC. Data is exported as a .CSV file for use with spreadsheet programs.
- Battery Status Indicator
- Error Messages
High Efficiency LED Light Source
An LED light source offers superior performance as compared to a tungsten lamp. LEDs have a much higher luminous efficiency, providing more light while using less power. They also produce very little heat, which could otherwise affect the optical components and electronic stability. LEDs are available in a wide array of wavelengths, whereas tungsten lamps are supposed to be white light (all wavelengths of visible light) but actually have a poor blue/violet light output.
High Quality Narrow Band Interference Filters
The narrow band interference filter not only ensure greater wavelength accuracy (+/- 1 nm) but are extremely efficient. The filters used allow up to 95% of the light from the LED to be transmitted as compared to other filters that are only 75% efficient. The higher efficiency allows for a brighter, stronger light source. The end result is higher measurement stability and less wavelength error.
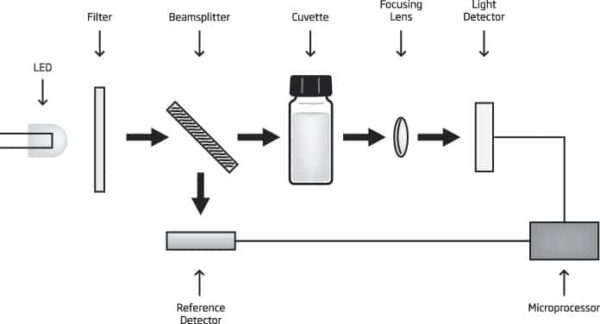
Reference Detector for a Stable Light Source
A beam splitter is used as part of the internal reference system of the HI-83300 photometer. The reference detector compensates for any drift due to power fluctuations or ambient temperature changes. Now you can rely on a stable source of light between your blank (zero) measurement and sample measurement.
Large Cuvette Size
The sample cell of the HI-83308 fits a round, glass cuvette with a 25 mm path length. Along with the advanced optical components, the larger size of the cuvette greatly reduces errors in rotation from the indexing mark of the cuvettes. The relatively long path length of the sample cuvette allows the light to pass through more of the sample solution, ensuring accurate measurements even in low absorbance samples.
Focusing Lens for Greater Light Yield
Adding a focusing lens to the optical path allows for the collection of all of the light that exits the cuvette and focusing the light on the silicon photo detector. This novel approach to photometric measurements cancels the errors from imperfections and scratches present in the glass cuvette eliminating the need to index the cuvette.
Suction Lysimeter HI83900
-For root level soil monitoring
-The perfect companion to the HI83325
-Monitor soil nutrients at the roots
The HI83900 suction lysimeter is built with porous ceramic cap connected to a transparent tube soil solution extraction. A rubber capillary is inserted in the tube passing through a rubber cap and reaching the ceramic tip.
The HI83900 series lysimeter is an ideal tool for collecting soil solution samples and then performing quantitative chemical analysis. In this way, the operator can easily monitor the level of nutrients such as ammonia, nitrate, phosphorous, potassium, sulfate, calcium and magnesium.

The ceramic tip of the lysimeter can be used in all types of soil. It is made of a sinterized material that does not react with nutrients in the soil.
Therefore, the soil solution collected is not affected by the chemical composition of the ceramic cap resulting in precise and reliable tests.
The HI83900 allows the extraction of a solution from the soil by creating a vacuum inside the sampler tube, that exceeds the soil water tension. This will establish an hydraulic gradient for the solution to flow through the porous ceramic cap and into the lysimeter tube. Typically, a vacuum of about -60 cb (centibar) should be drawn.
For better monitoring of soil solution composition throughout an entire growth period of a crop, at least two lysimeters should be installed in the root zone of a representative plant, on at the upper part and one in the lower part of the root zone.
For better measurement accuracy and repeatability, it is recommended to replicate installations in at least two more locations.

Spectrophotometer HI801
The HI-801 Iris is a sleek and intuitive spectrophotometer that allows for measurement of all wavelengths of visible light.
Customise your methods, take a wide range of measurements, and feel confident in your testing accuracy with iris.
Iiris features precise wavelength selection between 340 nm to 900 nm for complete method compliance and accuracy that is necessary in industries like professional laboratories, water treatment facilities, wineries, and more.
Results are consistent and accurate regardless of throughput with the high quality and uniquely designed optics system.
Customisation options include multiple cuvette shapes and sizes, custom calibration curves, and methods.

The 6 inch display screen is large and easy to read. The high contrast makes every character on the display stand out – even during outdoor use.
With its universal cuvette holder and auto-recognition feature, cuvette sizes can be changed when needed between round, square & vial.


No need for measurement conversions
Whether you are testing for chlorine or running enzymatic assays, our spectrophotometer will conveniently display results in the units that matter most to you. iris can measure in transmittance, absorbance, and concentration based on your need
Pre-programmed methods with the option to expand
iris comes pre-programmed with more than 80 commonly used chemical analysis methods to help you get started. Simply update these methods by connecting to a computer or flash drive.
Customise your iris with up to 100 personal methods. iris will guide you through the method creation process step-by-step. For added versatility, each method can include up to 10 calibration points, five different wavelengths, and up to five reaction timers.
Easily access your favourite methods directly from the home screen to save time.
Built in timers make measuring seamless. The countdown timer displays the time remaining until a measurement will be taken, ensuring consistent results between measurements and users. If you get stuck, the tutorial mode will walk you through the steps.


Designed for dynamic environments
iris’ compact profile and long-lasting battery make it easy to set up anywhere in your lab. The rechargeable lithium ion battery lasts for 3,000 measurements, or 8 hours – well over a full day of use out in the field.


Quality data without the hassle
Export your results with a USB drive or direct PC link organized by sample ID, method, or date range. Save data as a .pdf or .csv for maximum data integrity or flexibility – all without the use of specialized software.
Sort and share your data
Save data as a .pdf or .csv for maximum data integrity or flexibility. Have the freedom to choose the file format that is best for you.
Menu navigation that makes sense
Quickly navigate between screens with custom keys, and access your favourite methods directly from the home screen with our “favourite methods” feature.
All of your important information is easily visible
With a 6” display, the screen is large and easy to read. The high contrast makes every character on the display stand out even during outdoor use. The wide viewing angle allows for measurements to be seen from far away, so while working around the lab it is not necessary to hover over the meter to see the measurements.
Zero resistance with a capacitive touch pad
Menu buttons are part of the display. Built to be fully sealed and easy to clean, the meter recognises key touches even through gloves.
Change sample size easily
With its universal cuvette holder and auto-recognition feature, cuvette sizes can be changed when needed. The programmed cuvette size will be displayed on the screen every time you test to ensure that the proper path length is being used by the meter when calculating measurements for correct results.
Pocket Checkers
Hanna Checkers are small, handheld photometers that provide higher result accuracy than typical chemical test kits.
Available in 34 models offering dedicated parameter testing, each model in the range offers users improved digital technology in a compact design with easy to follow instructions making measurement quick and hassle-free.

Four steps. One click. Instant Readout.
- Zero’ the Checker®HC with your vial & water sample inside
- Take the vial out and add your reagent
- Place your vial into your Checker®HC
- Press the button and read the results







