Chemical composition of wine will change during maturation and aging. Organoleptic and chemical monitoring should be conducted regularly in order to reach optimum quality of wine during maturationin barrels or bottles. In the process of maturation, a lot of prosesses occur in wine: oxidation and reduction, decreas of wine acidity, formation of cream of tartar and biological degradation of acids. To prevent formation of unwanted chemical forms, sulphur dioxide is added in wine. Sulphur dioxide acts as a reduction, stabilisation and preservative agent. It has positive effect on bouquet formation, it protects oxidable substamces in wine against oxidation, prevents the loss of aromatic components and color of wine. SO2 has ability to bind oxygen and protect wine against enzymatic or non-ezymatic oxidation. Sulphur dioxide can go under changes during wine storage. Wine in upper parts of barrels can be more prone to oxidation and it showes lower amount of sulphure dioxide when compared to lower parts.


To understand what reducton is, firstly we have to talk about non-enzymatic browning.
Non-ezymatic browning is a process that produces the brown pigmentation in foods, but without the activity of enzymes. The two main forms of non-enzymatic browning are caramelization and the Maillard reaction (reaction of amino acids with reducing sugars such as glucose and fructose).


For reductones in wine, Maillard reactions are important. Sugars in grapes go through several reactions in Maillard reactions.The most important reaction is sugar dehydration.
Sugar dehydration occurs in two ways:
- furfural (low pH) is formed under acidic conditions
- reductants (reductons) (high pH) are formed in neutral or alkaline conditions
This can produce positively perceived aromas such as roasted (bread crust, coffee), caramel and malty, but also negative impressions such as bitter, burnt or rancid.
Method of measuring reductons in wine:
A wine sample (50 ml) was pipetted into an Erlenmeyer flask and 1% formaldehyde (1 ml) was added. The flask was sealed and its content was mixed. After 30 min, 16% H2SO4 (10 ml), Na2EDTA solution (1 ml) and starch solution (5 ml) were added using a graduated cylinder. The sample solution was titrated quickly with volumetric I2 solution [c (I2) = 0.01 mol/l] until blue colour persisting for 30 s was observed against the white background. The amount of standard I2 solution consumed in titration of reductones should be deducted in the calculation of molarity of free or total SO2 in wine. Reductone content was expressed as mg/l of ascorbic acid, or as mg/l of sulphur dioxide for the purpose of comparison with sulphur dioxide levels determined with a commercially available agent.
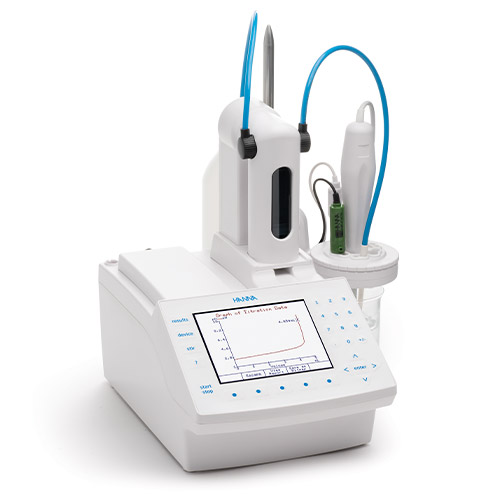
To measure reductons and sulphure dioxide in wine use:
HI931
Automatic Potentiometric Titrator
The HI931 Automatic Titrator is the answer to your dedicated titration needs. Fully customizable, the HI931 delivers accurate results and intuitive user experience, all in a compact package. Titrate for a variety of measurements at the push of a button including acids, bases, redox, and selective ions. No additional programming upgrades to purchase. The only things you need to start using the HI931 are a sensor and titrant.
- Small footprint so you can fully optimize your benchtop.
- Unmatched 40,000-step dosing pump for small volumes of titrant to help you achieve a very precise endpoint for greater consistency.
- Flexibility to store up to 100 methods.
Author:
Tajana Mokrović, mag.nutr.
Tajana Mokrović, mag.nutr.
Source:
Jančářová, I., JančářL, NáplavováA, & Kubáň, V. (2014). A role of reductones in the monitoring of sulphur dioxide content in wines during their maturation and storage Czech Journal of Food Sciences, 32(No. 3), 232–240. doi:10.17221/323/2013-cjfs
.